Partner with us for expert solutions that fit your unique challenge
We provide end-to-end solutions, guiding you from initial concept to full-scale production. Our ability to adapt to your unique requirements, coupled with our commitment to quality, sets us apart. By controlling the entire value chain, from CAD design to manufacturing in our ISO 8 cleanroom, we deliver tailored products that exceed your expectations.
Our rigorous project management approach is underpinned by deep technical expertise and a thorough understanding of regulatory landscapes.
By combining a structured methodology with continuous regulatory and technical monitoring, we proactively identify potential challenges and ensure strict compliance with CE and FDA standards. Every phase of your project is meticulously planned and tracked to guarantee a seamless and efficient process. Our commitment to quality and risk mitigation ensures that your medical device is brought to market successfully and on time.
Project Management


A proof of concept is a critical step in the product development lifecycle. By creating a functional prototype, we can validate the technical feasibility of your idea, refine product specifications, and gather valuable user feedback.
This iterative process allows us to de-risk your investment, accelerate time-to-market, and ensure that your product meets the needs of your target market. Our proof of concept process typically involves defining the scope, designing the prototype, conducting testing, and iterating based on the results.
Proof of concept


Our in-house design and development process ensures a final product that perfectly aligns with your goals.
By implementing a rigorous design freeze, we establish a clear milestone that marks the completion of the iterative design phase. Once all significant design changes have been incorporated and thoroughly tested, we finalize the design, creating a comprehensive set of design documents. This formal freeze acts as a blueprint for subsequent phases, including manufacturing, assembly, and quality assurance. By establishing a fixed baseline, we minimize the risk of scope creep, reduce the likelihood of costly rework, and enhance overall project efficiency. Moreover, a well-defined design freeze promotes clear communication and collaboration among all project stakeholders, ensuring a smooth transition from design to production. Ultimately, this disciplined approach enables us to deliver a high-quality product that meets your exact specifications within the agreed-upon timeframe.
Design and developpement


Our verification and validation (V&V) testing is rigorous and customized.
By employing specific test protocols, we meticulously evaluate every aspect of our products to ensure compliance with the highest standards. The results of these tests enable us to identify areas for improvement and drive continuous innovation. Through the use of advanced testing tools and realistic simulations, we can simulate extreme operating conditions, thereby guaranteeing the reliability and durability of our products.
V&V testing


Prior to any clinical evaluation on human subjects, our medical devices undergo a series of rigorous preclinical tests on animal models.
In partnership with the IHU Strasbourg, we meticulously assess the safety and efficacy of our products under controlled conditions. These tests, conducted in accordance with Good Laboratory Practices (GLP) and international regulations, enable us to identify potential adverse effects, evaluate biocompatibility, toxicity, and efficacy. Through this crucial step, we refine our developments, optimize product design, and ensure regulatory compliance before proceeding to clinical trials. This rigorous approach guarantees the safety of future patients and contributes to the advancement of biomedical research.
Preclinical testing
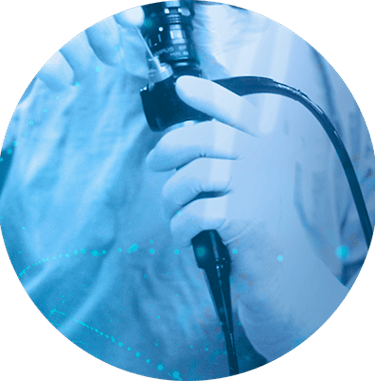
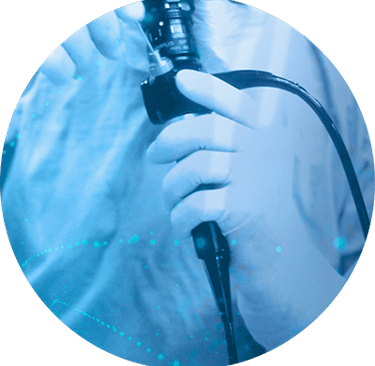
Leveraging our expertise in controlled environment production, we are capable of supporting our clients throughout the entire manufacturing process, from small-scale production to large-scale manufacturing in ISO 8 cleanrooms.
Our adaptable approach allows us to accommodate various production volumes and complexity levels, ensuring that our clients' specific needs are met.
Manufacturing


© 2024. All rights reserved.


Protomed SA
Contract medical device development in cardiovascular and minimally invasive surgery.
Join the team
